Arsenic is a toxic heavy metal that is found in mineral deposits throughout the Earth’s crust. Its presence is not unique to any particular region of the world, but it is thought that many millions of people might be exposed to arsenic in their drinking water in the USA, Bangladesh, China, Mexico, India and Nepal, Argentina, and elsewhere. Exposure to arsenic becomes even more worrisome if we were to consider water use in agriculture, food processing, and food preparation. So, to address this problem, we collaborated with Dr. Oliver Medvedik to invent an easy-to-use biosensor that could reliably and affordably indicate the presence of arsenic, and other toxins, in water.
Although it is a significant global health problem, Arsenicosis (arsenic poisoning) could be prevented if an affordable, easy-to-use device were available for testing water in the field. However, there are not many portable field-test devices that can reliably detect arsenic contamination at the World Health Organization’s recommended level of 10 ppb. The few field-test kits that can detect arsenic at this concentration (or lower) are complicated and expensive, and involve mixing chemical reagents. This means that skilled operators are usually needed to perform the tests in order to avoid flawed results, and the chemicals need to be disposed of properly. So, although these chemical kits are reasonably accurate, they have been of limited use in regions where highly skilled field-test operators are scarce, and where any test that costs more than a dollar is too expensive.
As an alternative, it has been shown that genetically modified organisms (GMOs) can function as biosensors that change color or emit light in response to environmental toxins. However, there are drawbacks to using GMOs as biosensors in consumer products. The organisms are difficult to store for long periods of time, even when freeze-dried, and it takes many hours for their revival and incubation at the right temperature and pressure to produce a warning signal. Battery-powered fluorometers are needed to read their low levels of bioluminescence, which is an added cost. There are also regulatory barriers that can impede the transport, use, and disposal of whole-cell GMOs, even if their only purpose is to serve as a small component in a handheld device.
Additionally, both the chemistry-based field kits and the GMO-based field kits are bulky and heavy to carry around, because they contain a number of necessary elements, compounds, and components. So, our challenge was to invent an alternative that is neither chemistry-based, nor GMO-based.
Instead of relying on chemical reagents or whole-cell GMO biosensors, our invention is a portable field-test device that includes an array of biomolecules printed on treated paper. When the paper-based device is wetted with contaminated water, its surface changes color, producing a warning that one can easily observe. If it does not change color, then the analyte is not present in the water sample and it is safe to use. Compared with current test kits, our invention is easier to make, transport, store, use, and dispose of.
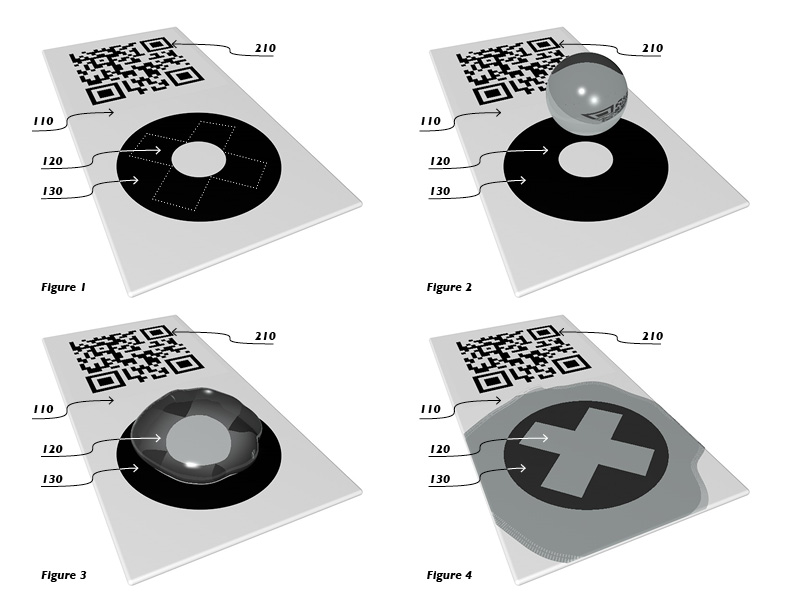
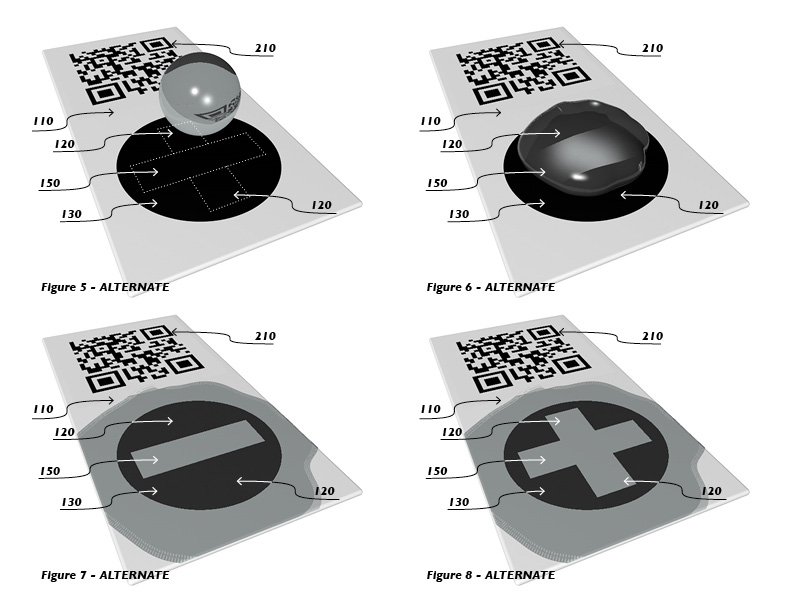
We created many embodiments of the invention that take the form of papers, sheets, cards, fibers, textiles, boards, tubes, ribbons, tapes, membranes and rods, but all of them have the same (or similar) features. The printable substrate for all of them is made of cellulose paper. Cellulose is the most abundant organic polymer on Earth. It is inexpensive, strong, flexible, hydrophilic, non-toxic, biodegradable, easily manufactured, and printable. All of them feature a color change that is enabled by a Protein-DNA biochemical interaction that rapidly responds to the presence of arsenic (and/or other target agents) in water samples. And all of the devices have a printed code tag that can be scanned with a smart phone or other electronic device, so a user can effortlessly record and report the test results to a recipient, or database.
The cellulose paper substrate is treated with a coating that enables us to bond the biochemical sensor to the cellulose surface. This bond coating immobilizes a layer of printed DNA biomolecules so that one end of the DNA is covalently bonded to the functionalized cellulose substrate. An array of chimeric ArsR proteins are then added to the other end of the DNA strands. This is possible because the ArsR proteins were designed so that, when they are a certain shape, the proteins have a strong affinity for the DNA strands. These ArsR proteins were also designed to have a nanoparticle secured to them, which gives them a particular color as a surface array.
When water is splashed on the surface and the ArsR proteins are exposed to arsenic in the water, the analyte bonds to the binding site of the ArsR, reducing the affinity between the proteins and the DNA molecules that they are joined to. Thus, any water sample that contains arsenic will cause the proteins to lose their attraction to the DNA and be washed away. This causes the nanoparticles to disperse with the proteins, causing a change in color that is readily observable. The nanoparticles might be made of gold, silver, titania, or germanium-coated gold. Other embodiments might include nanorods.
The biochemical sensor is surrounded by a printed boundary that is made with impervious ink of an unchangeable color. The color of the printed boundary matches the color of the biochemical sensor. It is this printed boundary that enables a well-defined graphic to emerge from the color change, as the ArsR proteins and nanoparticles are washed away from the printed boundary.
An alternative embodiment of the biochemical sensor inverts the architecture of the DNA and ArsR proteins [see Figure 12]. It positions the ArsR proteins so that they are covalently bonded to the bond coating, while it is the DNA array (with nanoparticles) that are washed away in the presence of a target agent, such as arsenic. The invention can be integrated into other objects, such as: tape rolls, smart phones, and stand-alone electronic devices. And it can be used for other forms of analyte monitoring for blood & body, air & environment, chemical concentrations, manufacturing, disaster spills, attacks, etc.
Project Team:
- Oliver Medvedik & Peter Yeadon, Project Leads
- Ann Dinh, Marty Laurita, Anne Morrill, Ilker Öztop